The Lowe Syndrome Trust UK, Dent Disease Foundation USA and The Lowe Syndrome Association USA have each awarded grants of €20,000 towards a pilot clinical study led Professor Francesco Emma, Head of the Nephrology unit at the Bambino Gesù Children’s hospital in Rome, Italy. The first patients were enrolled into this Phase 2 clinical trial of alpelisib in Dent disease 2 in June. Dr Emma said, “This is the first trial in patients with Dent disease due to OCRL1 mutations, based on repurposing a drug that targets the underlying mechanism causing the disease. It aims to find out if the drug improves abnormal kidney function”.
Dent disease (type 2) is a rare condition due to inactivating mutations in the OCRL1 gene that affects males. The most common manifestation of the disease is dysfunction of the proximal tubule of the kidney, which manifests as low-molecular-weight (LMW) proteinuria. The disease may be difficult to recognise and can lead to life-threatening complications and kidney failure at a young age. Dent disease can be cured with kidney transplant but there is a shortage of available organs, and a transplant requires lifelong immunosuppression - so a drug treatment would be a significant step forward. Jill Goodrich, Co-Executive Director of the Dent Disease Foundation said, ‘If a treatment is available, we hope that testing for LMW proteinuria will become more widespread to the benefit of not only Dent disease 2 patients but also the other genetic causes of Dent disease. The progress to chronic kidney disease is a burden that affects whole families, and we look forward to hearing about the progress of the research’.
Lowe syndrome is caused by other mutations in the same OCRL1 gene and is a more severe multi-organ condition causing congenital cataracts, intellectual disability, autism-like symptoms and seizures amongst other symptoms. These disorders have a profound effect on patients and their families. Longstanding supporters of the Lowe Syndrome Trust include patrons Jonathan Ross OBE, Penny Lancaster Stewart, and Tony Hadley MBE. Penny Lancaster, Patron of the Lowe Syndrome Trust commented “We are delighted that the Lowe Syndrome Charity is supporting this clinical trial and hopeful that this will be a major breakthrough in the treatment of Dent 2 and Lowe Syndrome and transform the lives of the affected families”.
Image Credit: Theresa Haugen. Lead clinician Francesco Emma (front right), discussing the ALPEDENT trial with (anticlockwise) Robert Nussbaum, Jenny Gallop, Andrew Thomas, Joseph Laycock and Jeri Kubicki from the Lowe syndrome patient associations at a research meeting in 2023.
The trial has been made possible by an international consortium of scientists, clinicians and the patient groups that are funding the study. The drug compound being used - alpelisib - was identified by a collaboration between Dr Jennifer Gallop’s laboratory at the Gurdon Institute and Department of Biochemistry, University of Cambridge UK and Professor Olivier Devuyst’s laboratory at the Institute of Physiology, University of Zurich, Switzerland.
Dr Gallop gave a keynote lecture at the Lowe Syndrome Association conference on 29 June in Cincinnati, Ohio, USA. She talked about the route to treatments and the significance of the trial in Dent 2 patients for their goal of a meaningful treatment by 2030. Rod Ankrom, whose son lives with Lowe syndrome commented, “We have been coming to LSA conferences for 34 years, and for the research at first there were just guesses, and after decades of work, there now really might be a treatment coming down the line”. Jeri Kubicki, President of the Lowe Syndrome Association said, ‘Our boys experience Lowe syndrome in different ways, at different stages. We are exploring multiple avenues and are excited to determine what this clinical trial tells us’.
Image: Dr Gallop giving her lecture at the 2025 LSA conference
Dr Gallop and her team at the University of Cambridge have been working on the fundamental science behind the trial for over 15 years. Their research, funded by the Wellcome Trust and Lowe Syndrome Association is on the actin cytoskeleton that is in all cells in our body and important for all functions, like pumping our heart and supporting our immune system. When Gallop worked out that specific lipid molecules in cell membranes are important in controlling the actin cytoskeleton, she saw that there was a link with Lowe syndrome and Dent disease 2, which causes alterations to these lipids. Gallop said: “And excitingly, we realised there’s an existing drug that might be able to help”.
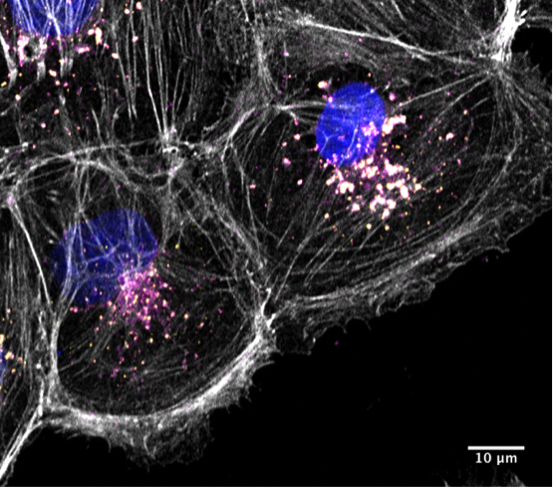
Image Credit: Jonathan Gadsby. Human Kidney 2 cell showing actin assemblies around endosomes in OCRL1 deficient cells (actin white, endosomes in yellow and pink and nuclei in blue).
At that time, Dr Gallop reached to the group of Professor Olivier Devuyst (University of Zurich), who has done pioneering work on genetic disorders of the kidney proximal tubule, including the cellular mechanisms and models of Dent disease. Devuyst is co-directing the ITINERARE Research Priority Program in Zurich, which works at translational programs to accelerate drug discovery for rare diseases. Devuyst, Berquez and colleagues were able to test the drug in model kidney cells, and then in a humanised mouse model of Dent disease 2 – that they had just characterised. Working together, the team found that alpelisib did work to rebalance the actin cytoskeleton and improve kidney function in lab models of the diseases. Professor Devuyst commented, ‘This pioneering trial builds on decades of research establishing the crucial role of endolysosomal trafficking in kidney proximal tubule cell function. It also provides a rare opportunity to repurpose a drug that has been decisively useful in another set of rare disorders sharing problems with the small lipids’. These fundamental insights were detailed in a joint publication (Berquez M et al. Kidney Int 2020, with Editorial Commentary), paving the way for the preparation of the Phase 2 clinical trial.
The trial is due to report in October 2026 and if it shows that alpelisib is effective in the Dent disease 2 participants, the hope is that it will also be beneficial to Lowe syndrome patients suffering with kidney disease.